Exhaustive follow-up of patients wearing Total Hip Prostheses with Dual Mobility Cups.
Collective information note relating to the SNDS post-registration study for the exhaustive follow-up of patients with Total Hip Prostheses with Dual Mobility Cups (EPI PTH CDM).
In accordance with the provisions of Article 14 of the RGPD, this collective information notice describes the measures implemented in the context of studies that do not allow for individual information and require access to data from the National Health Data System (SNDS).
- Data controller:
X.NOV MEDICAL TECHNOLOGY SA with registered office at Rue d'Airmont 7, CH-2900 PORRENTRUY, represented by its European representative X. NOV located at 14 rue du Chêne sec, - ZAC les Guinnottes 2, 70400 HERICOURT, registered in the VESOUL Trade and Companies Register under no. 448 101 626, is carrying out a study assessed to be in the public interest by the Comité d'Expertise pour les Recherches, les Etudes et les Evaluations dans le domaine de la Santé (CESREES) on December 10, 2020 (for more information, visit https://www.health-data-hub.fr/outil-de-visualisation). This study is delegated to IQVIA Opérations France, located at 17 bis Place des Reflets 92400 Courbevoie, registered with the Nanterre Trade and Companies Register under number 347 939 415, which is responsible for processing the data.
- Contact details of the Data Controller's representative: eu.dpo@iqvia.com
- Purpose:
The main objective is to quantify and compare the overall survival rates of total hip prosthesis wearers (duration of implantation without revision) and dislocation rates in the group with dual mobility cups versus single mobility cups, overall and according to revision risk factors in a national post-registration study.
Legal basis: In accordance with Article 6 of the RGPD and Article 5 of the Loi Informatique et Libertés, the processing carried out as part of this study is based on the public interest pursuing an objective of research, studies, evaluation and innovation in healthcare. This study, shared by some twenty manufacturers, was set up at the request of the CNEDiMTS (opinion of January 24, 2017) for the renewal of the registration of these products on the List of Reimbursable Products and Services.
In accordance with Article 9 of the RGPD, the processing of this personal data concerning health responds to scientific research purposes.
On April 2, 2020, the Comité Ethique et Scientifique pour les Recherches, les Etudes, et les Évaluations en Santé (CESREES) declared the study to be of public interest (number 1502850).
The study was authorized by the CNIL in accordance with article 66 of the French Data Protection Act no. 78-17 of January 6, 1978, as amended (Decision DR-2020-210; July 10, 2023).
- SNDS data categories used:
Data extracted from SNIIRAM (Système National d'Information Inter Régimes de l'Assurance Maladie), PMSI (Programme de Médicalisation des Systèmes d'Information) databases held by the national health insurance fund (CNAM) between 01/01/2012 and 31/12/2023.
The data processed in the SDNS are data relating to hospitalizations (illness responsible for hospitalization and associated comorbidities) and/or reimbursed care provided in the community (medical consultation, delivery of treatments, medical procedures, etc.), as well as socio-demographic data (age/year of birth, gender, etc.).
In accordance with the French Public Health Code (CSP), the personal data contained in these databases cannot be used to identify the individuals to whom they relate.
- Data retention period: 3 years after availability.
- Data controller and data recipients :
Data are made available by CNAM on the CNAM secure IT portal for the designated implementer, IQVIA.
- Data transfer: This data will not be transferred outside the European Union.
- Exercising rights and making claims:
In accordance with the RGPD and the French Data Protection Act (Loi Informatique et Libertés), you have, as a matter of principle, a right of access, rectification, limitation and deletion of the data concerning you processed within the framework of the Study, which you may exercise by contacting the Data Protection Delegate of IQVIA Operations France Ms. Barbara BRESSOLLES, at the following address eu.dpo@iqvia.com.
We remind you that for general opposition to any re-use of SNDS data, the provisions of article R 1461-9 of the CSP relating to the terms and conditions for exercising rights stipulate that rights of access, rectification and opposition may be exercised with the Direction de la Plateforme des Données de Santé (Health Data Hub, https://www.healthdata-hub.fr/contact) or with the director of the compulsory health insurance organization to which you are attached.
You also have the right to lodge a complaint with the Commission Nationale de l'Informatique et des Libertés (CNIL) online or by post at CNIL - Service des Plaintes - 3 Place de Fontenoy - TSA 80715 - 75334 PARIS CEDEX 07.

 on your implant card labels. Then read the relevant row to find out how long it will last.
on your implant card labels. Then read the relevant row to find out how long it will last.  on your labels.
on your labels. 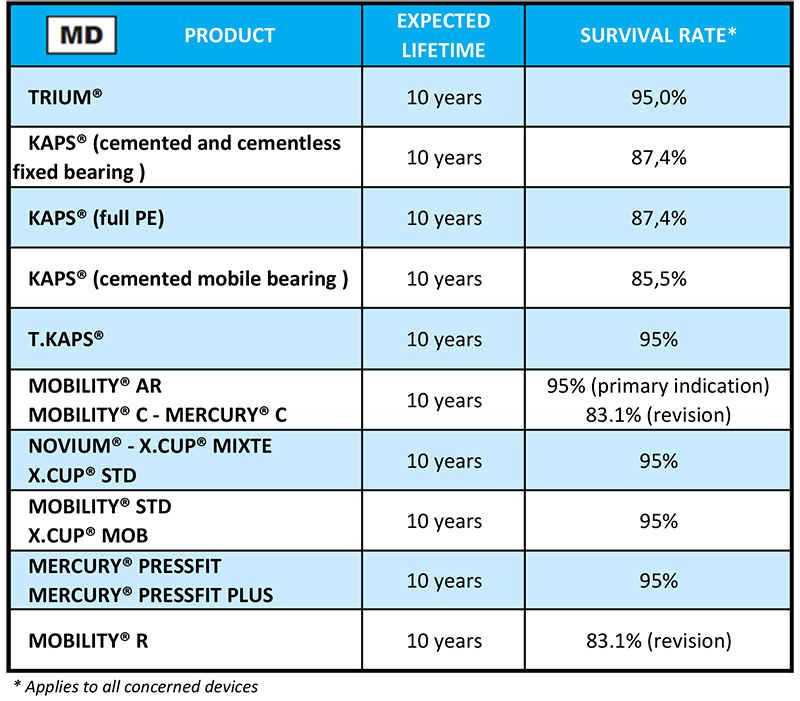
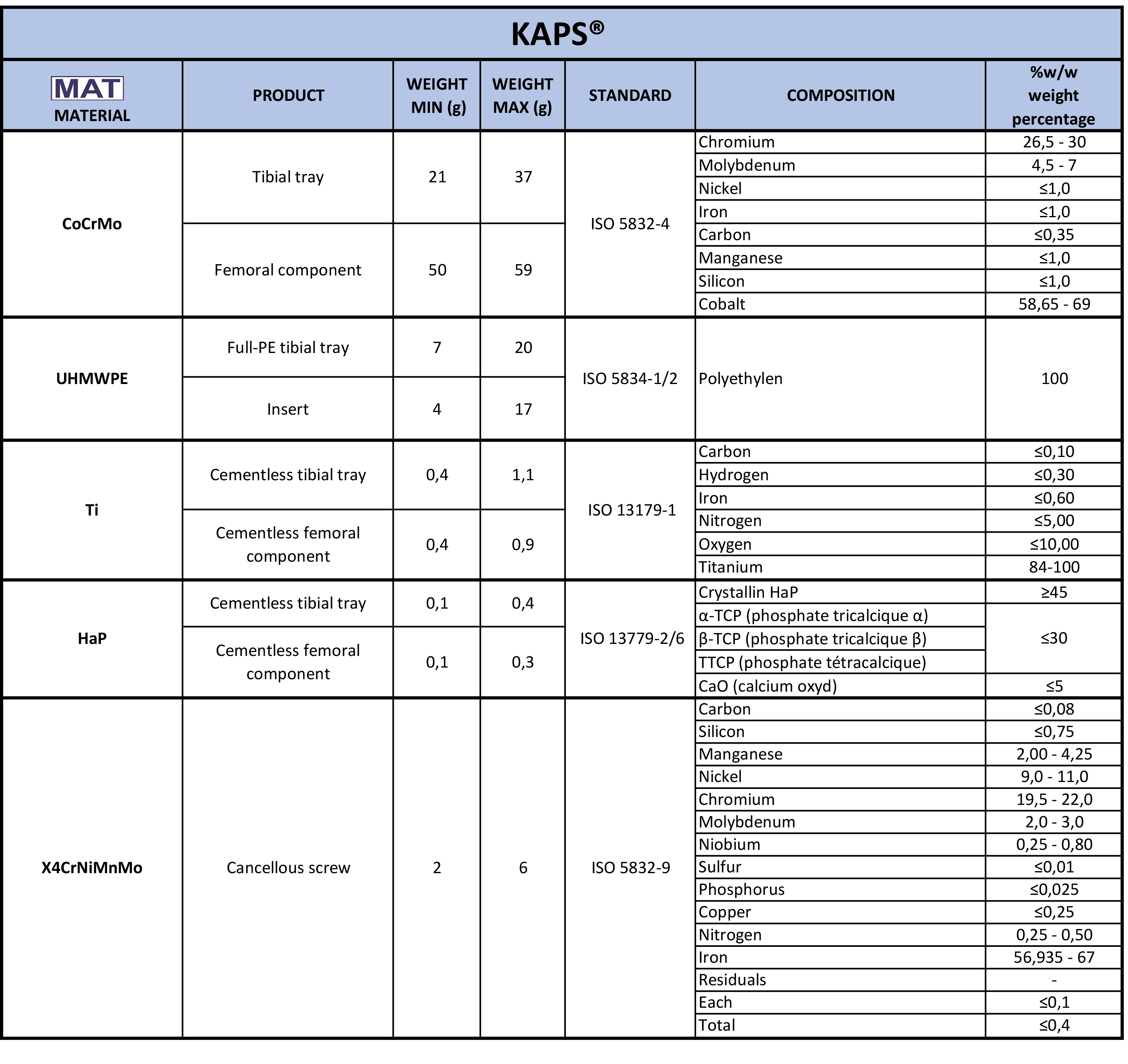
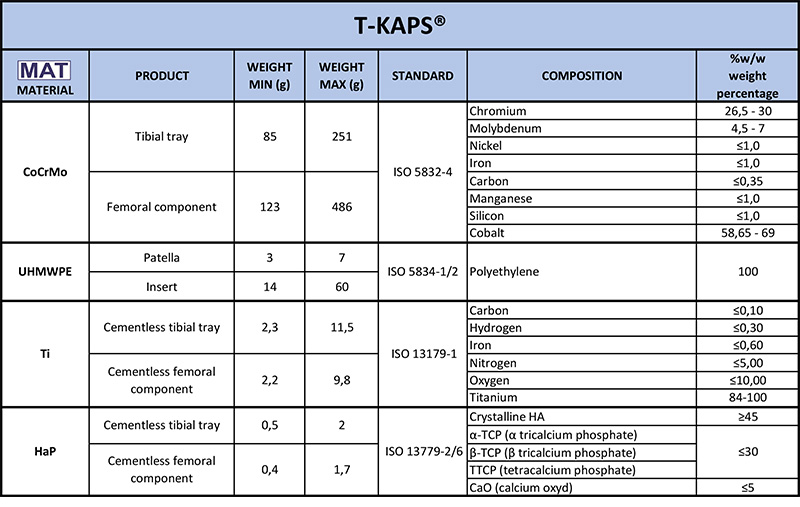
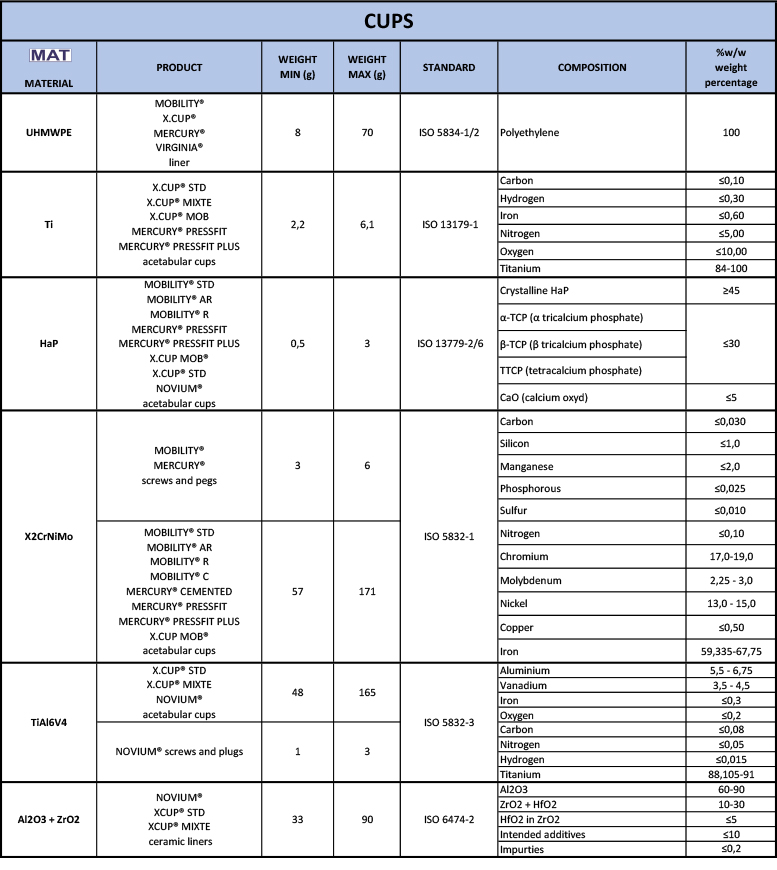
 logos on your implant card labels. Then read the relevant row to find out what it is made of.
logos on your implant card labels. Then read the relevant row to find out what it is made of.  of your labels.
of your labels.